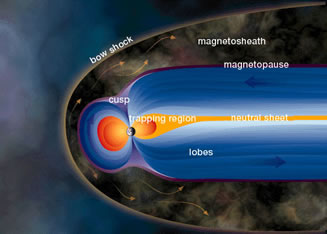
Charged Particle Motion in Earth's Magnetosphere
Auroral Colors and Spectra
When the aurora is faint, it appears white to the unaided eye. As its brightness increases, color vision starts to work, and the characteristic pale green hue of the aurora becomes visible.

With more intensity, a bright green color is visible in the lower regions, and a faint red glow can be discerned at high altitude.

When the aurora is very energetic and very bright, a deep red lower border appears below the green.

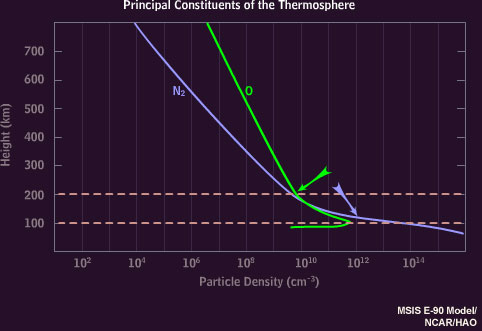
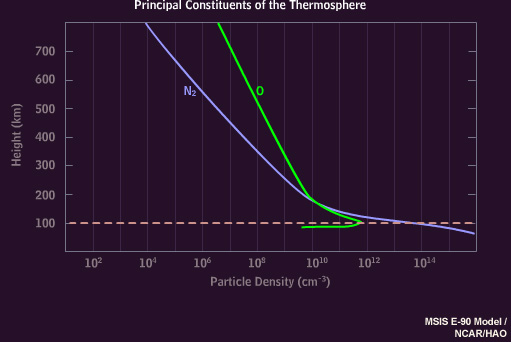
The atmosphere above 100 kilometers consists mainly of nitrogen molecules and oxygen atoms, with more nitrogen at 100 kilometers transitioning to proportionally more oxygen at 200 kilometers.
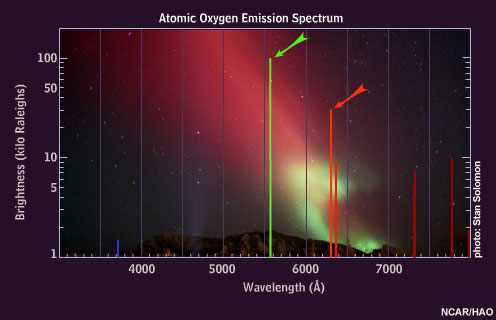
When oxygen atoms are excited, they emit light of various colors, but the brightest are the red and green emissions.
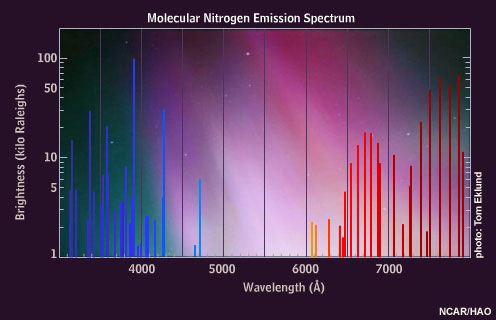
Excited nitrogen molecules emit red, blue, and violet light. The interplay of oxygen and nitrogen and their proportional changes with altitude cause the typical appearance of auroral colors.
Some of the excited nitrogen molecules also interact with the atomic oxygen, causing an additional green emission at a wavelength of 558 nanometers. This emission, called the "auroral green line," is seen throughout the aurora down to about 100 kilometers, giving the aurora its dominant green appearance.
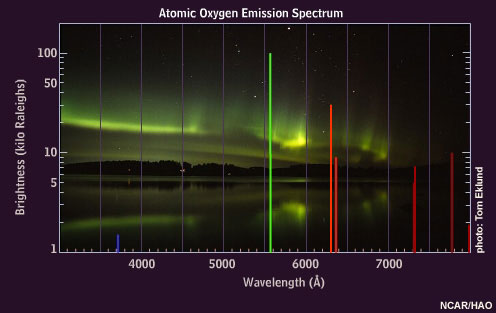
Oxygen atoms emit 630 nanometer "auroral red line" light only at very high altitudes because these atoms are de-excited by collisions with nitrogen molecules below about 150 kilometers.
The auroral green line disappears below 100 kilometers, where little atomic oxygen exists.
When highly energetic particles reach low levels, they trigger the red, blue, and violet emissions of molecular nitrogen, observed as a deep red or magenta border at the lower edge of auroral curtains. This dramatic effect is enhanced by the rapid motion associated with these very energetic displays. It is also possible to see waves of green light "chasing" the magenta, an effect caused by the time lag between excited oxygen and nitrogen. Oxygen atoms persist for about one second in their unstable state before decay, while the nitrogen molecule emissions are instantaneous.