Click on image for full size
Windows to the Universe original image
Density of Ocean Water
The density of pure water is 1000 kg/m3. Ocean water is more dense because of the salt in it. Density of ocean water at the sea surface is about 1027 kg/m3.There are two main factors that make ocean water more or less dense than about 1027 kg/m3: the temperature of the water and the salinity of the water. Ocean water gets more dense as temperature goes down. So, the colder the water, the more dense it is. Increasing salinity also increases the density of sea water.
Less dense water floats on top of more dense water. Given two layers of water with the same salinity, the warmer water will float on top of the colder water. There is one catch though! Temperature has a greater effect on the density of water than salinity does. So a layer of water with higher salinity can actual float on top of water with lower salinity if the layer with higher salinity is quite a bit warmer than the lower salinity layer.
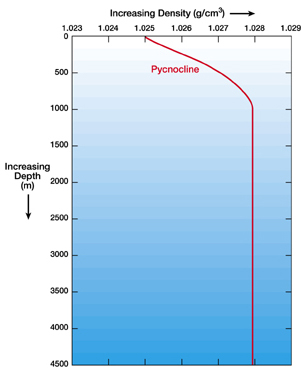
The temperature of the ocean decreases and decreases as you go to the bottom of the ocean. So, the density of ocean water increases and increases as you go to the bottom of the ocean. The deep ocean is layered with the densest water on bottom and the lightest water on top. Circulation in the depths of the ocean is horizontal. That is, water moves along the layers with the same density.
The density of ocean water is rarely measured directly. If you wanted to measure the density of ocean water, you would have to collect a sample of sea water and bring it back to the laboratory to be measured. Density is usually calculated using an equation. You just need to measure the salinity, temperature and pressure to be able to find density. These measurements are often made with a CTD instrument, where the instrument is placed in the ocean water from a ship or a platform.