Click on image for full size
Credit: UNC
Acidic Ocean Water Impacts Corals and Other Marine Life
Increasing amounts of carbon dioxide are released into the atmosphere from burning of fossil fuels. Some of that carbon dioxide makes its way into the world’s oceans. This changes the chemistry of seawater, lowering its pH, making it more acidic, which could have a large impact on marine life in the future.
Marine creatures such as corals, clams, snails, and many types of algae and plankton build their skeletons and shells from calcium carbonate. These creatures get the chemical building blocks they need to form the calcium carbonate mineral of their skeletons from seawater. As seawater gets more acidic with more carbon dioxide dissolved in it, these creatures might have a harder time making their skeletons and shells.
Calcium carbonate mineral dissolves in acid. Try it out for yourself. Put a clam shell (one that you don’t want to keep) into a container of vinegar and wait. Vinegar is an acid. Within a few hours will notice that your clam shell is disappearing. The calcium carbonate that makes up the shell is dissolving into the acidic vinegar.
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at 25 °C (77 °F). Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline.
Seawater's pH is not dropping as low as vinegar. Vinegar has a pH of about 3. The pH of seawater varies between 7 and 8, so it is a little more basic than neutral. Since the start of the Industrial Revolution, pH of seawater has dropped about 0.1. In the next century it is expected to drop another 0.1-0.35. But scientists suspect that even these small changes can make a big difference to the creatures that need to build their shells.
Because reef corals build massive structures from calcium carbonate, and because those structures become a home to diverse communities of marine life, the impact of increasing acidification on corals is of particular interest to many scientists.
The current research indicated that for a doubling of the partial pressure of carbon dioxide (pCO2), rates of calcification decreased in corals an average of 30%. The rate is affected by many other factors besides the concentration of CO2 dissolved in the water. The temperature, light, and nutrients all affect calcification rates too.
Lower rates of calcification will likely impact marine food webs, possibly changing the biodiversity of the ocean.
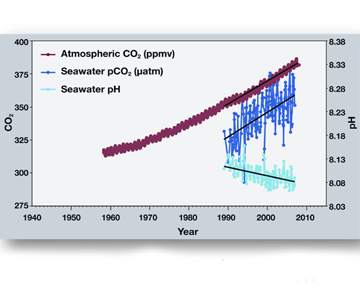
Sometimes a picture is worth a thousand words. This image shows the correlation between rising levels of carbon dioxide (CO2) in the atmosphere at Mauna Loa with rising CO2 levels in the nearby ocean at Station Aloha. As more CO2 accumulates in the ocean, the pH of the ocean decreases.
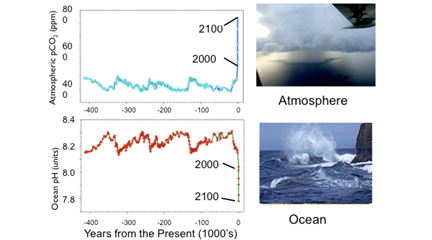
Another helpful image is the following comparative graph set. The top graph shows the variation of CO2 in the Earth's atmosphere over the last 400,000 years. You can see the blue line goes up and down with natural variations in levels of CO2 until just before Present where CO2 levels spike drastically upwards due to human contribution of the gas. Projected levels of CO2 are shown for 2100. On the bottom graph, the ocean's pH is shown. You can see that this inversely varies with the atmospheric CO2. You can see natural variations of the ocean's pH until just before Present, where the pH plummets from natural levels. Projected pH level is around 7.8 in 2100, much lower (more acidic) that the ocean is now.