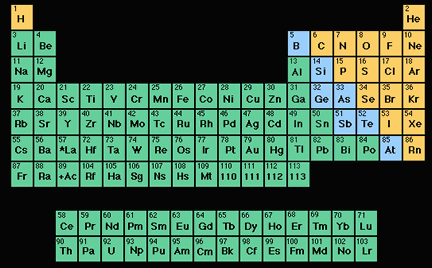
The periodic table of the elements.
Click on image for full size
L.Gardiner/Windows to the Universe
The Periodic Table of the Elements
Everything you see around you is made of tiny particles called atoms, but not all atoms are the same. Different combinations of protons
, neutrons
and electrons
make different types of atoms and these different types are called elements.
The picture to the left shows the periodic table of the elements. Scientists made this table as a way to organize all the elements that have ever been found or created.
Each element has its own symbol. For instance, the upper left square of the table is labeled ‘H’. The ‘H’ is the symbol for an element called hydrogen.
Notice how each element in the table has its own number. This is called the atomic number and shows us how many protons are in the nucleus of each atom of the element. It also tells us how many electrons are in a neutral atom of the element. For instance, the ‘1’ listed in hydrogen’s square indicates that each atom of hydrogen has one proton and one electron.
Each row in the table tells how many shells around the nucleus an element’s atoms have to hold electrons. For example, hydrogen (H), in the top row has one electron shell while Lithium (Li) in the row below, has two electron shells.
Of all the elements in the periodic table, only the first 92 are naturally found, while the others are synthetically made. The 92 natural elements are the ingredients used to make everything we find on Earth.
Last modified April 29, 2003 by Lisa Gardiner.
You might also be interested in:
An element (also called a "chemical element") is a substance made up entirely of atoms having the same atomic number; that is, all of the atoms have the same number of protons. Hydrogen, helium, oxygen,
...more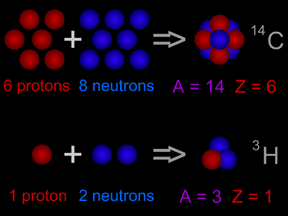
The atomic number of an atom tells us how many protons are in the nucleus of that atom. Why is that important? The chemical properties of an element are determined by the number of electrons in its atoms,
...more
Chemistry is the study of matter, energy, and their interactions. Chemists study the composition of substances, their properties, and how they react with each other under varying circumstances. Indeed,
...more
Minerals occur naturally on rocky planets and form the building blocks of rocks. They are non-living, solid, and, like all matter, are made of atoms of elements. There are many different types of minerals
...more
Even though there are 92 elements that naturally occur, only eight of them are abundant in the rocks that make up the Earth’s outer layer, the crust. Together, these 8 elements account for 98.5% of the
...more
Intrusive igneous rocks, also called plutonic rocks, form deep below the Earth’s surface when magma, or molten rock, rises into a crack or an underground chamber within the Earth. The chamber is a little
...more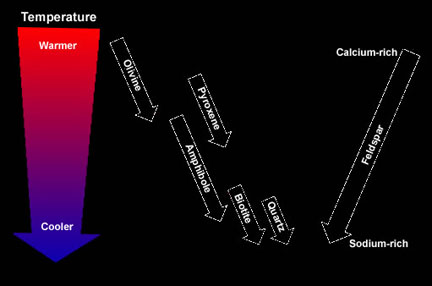
As magma cools slowly, elements within it become chemically bonded forming crystals of minerals. However, not all minerals form at the same time during the cooling process. Some minerals crystallize when
...more













