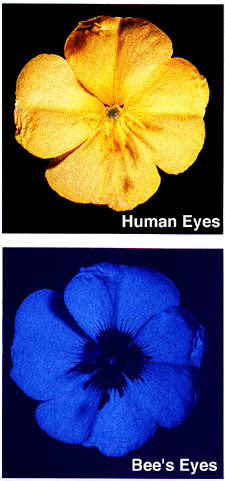
Image courtesy Dr. Jeremy Burgess, Science Source/Photo Researchers.
Ultraviolet (UV) Radiation
Ultraviolet (UV) "light" is a type of electromagnetic radiation. UV light has a shorter wavelength than visible light. Purple and violet light have shorter wavelengths than other colors of light, and ultraviolet has even shorter waves than violet does; so ultraviolet is sort of "purpler-than-purple" light or "beyond violet" light.
Ultraviolet radiation lies between visible light and X-rays on the electromagnetic spectrum. UV "light" has wavelengths between about 380 and 10 nanometers. The wavelength of violet light is around 400 nanometers (or 4,000 ┼). Ultraviolet radiation oscillates at rates between about 800 terahertz (THz or 1012 hertz) and 30,000 THz.
The ultraviolet spectrum is sometimes subdivided into the near UV (380 to 200 nanometer wavelengths) and extreme UV (200 to 10 nm wavelengths). Normal air is largely opaque to UV with wavelengths shorter than 200 nm (the extreme UV range); oxygen absorbs "light" in that part of the UV spectrum.
In terms of impact on the environment and human health (and choosing sunglasses!), it can be useful to subdivide the UV spectrum in a different way, into UV-A ("blacklight" or Long Wave UV with a 380 to 315 nm wavelength), UV-B (Medium Wave at 315 to 280 nm), and UV-C (the "germicidal" or Short Wave UV that ranges from 280 to 10 nm).
Earth's atmosphere prevents most UV radiation from space from reaching the ground. UV-C is entirely screened out by stratospheric ozone at around 35 km altitude. Most UV-A does reach the surface, but UV-A does little genetic damage to tissues. UV-B is largely responsible for sunburn and skin cancer, though it is mostly absorbed by ozone before reaching the surface. Levels of UV-B radiation at the surface are especially sensitive to levels of ozone in the stratosphere.
Ultraviolet radiation causes sunburn. It is used to sterilize glassware used in medicine and biological research.