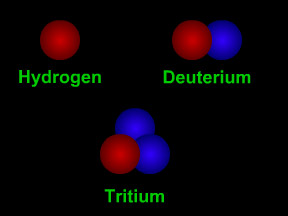
Hydrogen has three isotopes. The nucleus of a "normal" hydrogen atom has one proton (red) but no neutrons (blue). Hydrogen's other isotopes are deuterium (1 proton + 1 neutron) and tritium (1 proton + 2 neutrons).
Click on image for full size
Original artwork by Windows to the Universe staff (Randy Russell).
Isotope
Isotopes are different "versions" of an element. All atoms of an element have the same number of protons. All hydrogen atoms have one proton, all carbon atoms have 6 protons, and all uranium atoms have 92 protons. However, atoms of an element can have different numbers of neutrons. Most carbon atoms have 6 neutrons, but some have 7 neutrons.
Scientists use special "codes" to write the names of isotopes. One isotope of carbon has 8 neutrons. It has an atomic mass of 14 (6 protons + 8 neutrons). The "code" for this isotope is carbon-14 or 14C. Different isotopes of the same element behave almost exactly the same way in chemical reactions. For example, most oxygen is the isotope oxygen-16. Oxygen-18 is a rare isotope. However, adding two hydrogen atoms to one oxygen atom still makes water (H2O), even if we use 18O instead of 16O. Some isotopes are radioactive, but others are not. Radioactive isotopes can "decay" by giving off radiation.
Where do different isotopes come from? Astronomers think the only elements created in the Big Bang were various isotopes of hydrogen, helium, and probably lithium, beryllium and boron. Supernova explosions created the rest of the elements, including most of their isotopes. Some isotopes form when high-energy cosmic rays crash into atoms in our atmosphere.
You might also be interested in:
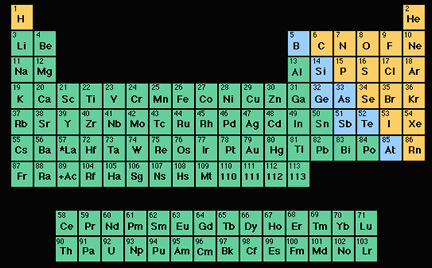
An element (also called a "chemical element") is a substance made up entirely of atoms having the same atomic number; that is, all of the atoms have the same number of protons. Hydrogen, helium, oxygen,
...more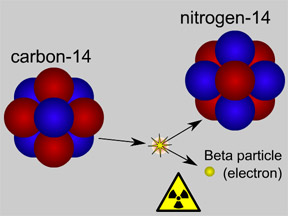
Some materials are radioactive. They give off radiation. When an atom of a radioactive substance gives off radiation, it becomes a new type of atom. This change is called radioactive decay. There are two
...more
The text for this level hasn't been written yet. Please check the "Intermediate" or "Advanced" level of this page (click on the bar near the top of this page).
...more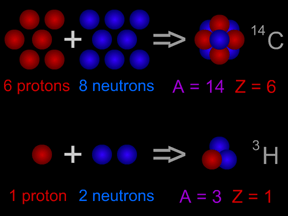
Carbon-14 is an isotope of the element carbon. All carbon atoms have 6 protons in their nucleus. Most carbon atoms also have 6 neutrons, giving them an atomic mass of 12 ( = 6 protons + 6 neutrons). Carbon-14
...more
One way scientists measure the size of something is by its mass. Mass is sort of like weight. Scientists can even measure very, very tiny things like atoms. One measure of the size of an atom is its "atomic
...more
Every atom has a nucleus. The nucleus has protons and neutrons in it. Scientists have a special name for the number of protons in an atom. They call it the "atomic number". There are almost 100 different
...more
Carbon-14 dating (also called "radiocarbon dating") is used to determine the age of materials that contain carbon that was originally in living things. It is often used in archeology and some
...more