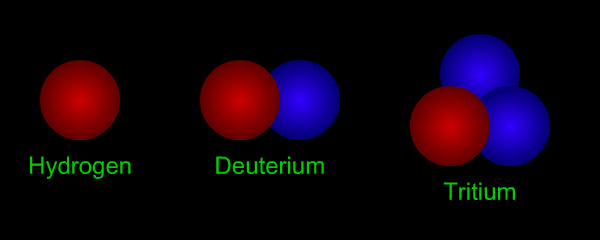

Hydrogen has three isotopes. The nucleus of a "normal" hydrogen atom has one proton (red) but no neutrons (blue). Hydrogen's other isotopes are deuterium (1 proton + 1 neutron) and tritium (1 proton + 2 neutrons). Most hydrogen (more than 99%) is the normal isotope without any neutrons.
Original artwork by Windows to the Universe staff (Randy Russell).