This picture shows radioactive decay of a carbon-14 atom. The carbon atom gives off a beta particle of radiation. The carbon atom turns into a nitrogen atom.
Click on image for full size
Original artwork by Windows to the Universe staff (Randy Russell).
Carbon-14 Dating
Carbon-14 dating (also called "radiocarbon dating") is used to determine the age of materials that contain carbon that was originally in living things. It is often used in archeology and some types of biology. Living creatures ingest carbon. Plants (and other autotrophs) take in carbon dioxide gas from the atmosphere during photosynthesis. Animals (and other heterotrophs) get their carbon by eating plants or other animals, from decaying organic matter, or from other similar sources.
Some of the carbon is a radioactive isotope called carbon-14 (14C). When the creature dies, it stops ingesting carbon. The radioactive 14C gradually undergoes radioactive decay, transforming it into nitrogen, and therefore gradually "disappears". Scientists can study samples from the once-live creatures' remains to see how much radioactive 14C, as compared to the normal isotope of carbon (carbon-12 or 12C), is still around. This tells the scientists how long ago the organism died.
14C has a half-life of 5,730 years, meaning that after about five thousand years about half of the 14C will decay and turn into nitrogen. After several half-lives, too little 14C will remain in a sample for it to be useful for dating. Radiocarbon dating is therefore only useful for samples with ages of less than about 65,000 to 80,000 years.
You might also be interested in:

Organisms that are able to "make their own food" are called autotrophs, meaning "self-feeders". Some examples of autotrophs are plants and algae (shown in the picture). Both plants and algae use photosynthesis
...more
There are many different kinds of plants. Some have big leaves. Some have small leaves. Some even have flowers. All plants make their own food. When sunlight hits the leaves of a plant, photosynthesis
...more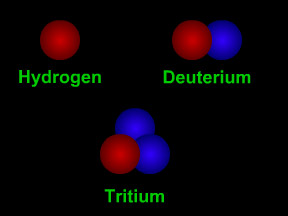
Isotopes are different "versions" of an element. All atoms of an element have the same number of protons. All hydrogen atoms have one proton, all carbon atoms have 6 protons, and all uranium atoms have
...more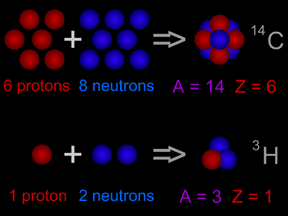
Carbon-14 is an isotope of the element carbon. All carbon atoms have 6 protons in their nucleus. Most carbon atoms also have 6 neutrons, giving them an atomic mass of 12 ( = 6 protons + 6 neutrons). Carbon-14
...more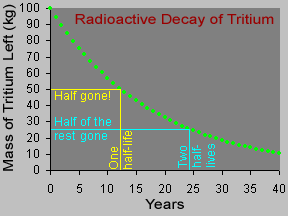
Some materials are radioactive. Their atoms give off radiation. When an atom gives off radiation, it turns into a different kind of atom. That is called radioactive decay. Some atoms decay very quickly,
...more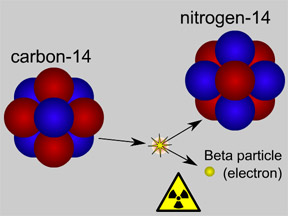
Some materials are radioactive. They give off radiation. When an atom of a radioactive substance gives off radiation, it becomes a new type of atom. This change is called radioactive decay. There are two
...more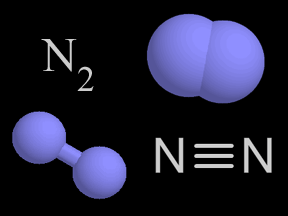
There is more nitrogen gas in the air than any other kind of gas. About four out of five of the molecules in Earth's atmosphere is nitrogen gas! A molecule of nitrogen gas is made up of two nitrogen atoms.
...more