Introduction to Ozone Article
*Please note: This reading comes from the LEARN web site and has been copied here with permission for the purposes of this specific reading activity. Please see the LEARN site for more great activities regarding Ozone and more.*Introduction
The Ozone Hole. The Ozone Hoax. Pollution. Skin Cancer. The topic of ozone makes headlines on a regular basis, but why does a single molecule merit such media coverage? How important is the ozone in our atmosphere and why are scientists so concerned about its increase near the surface of the earth and its disappearance higher up in the atmosphere?
First things first - what is ozone? Ozone is made of three oxygen atoms (O3). The oxygen we find in our atmosphere is made up of two oxygen atoms (O2). Because of its chemical formulation, a single atom of oxygen (O) is unstable. That is, it wants to combine with something else. That is why oxygen is almost always found in pairs, in its O2 (diatomic) form, where it is more stable. O3 is less stable than O2, because it wants to return to the diatomic state by giving up an oxygen atom.
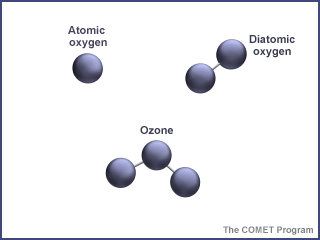
When enough ozone molecules are present, it forms a pale blue gas. It is an unstable molecule that readily combines with other atoms. Ozone has the same chemical structure whether it is found in the stratosphere or the troposphere. Where we find ozone in the atmosphere determines whether we consider it to be Dr. Jekyll or Mr. Hyde.
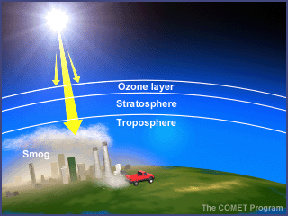
In the troposphere, the ground-level or "bad" ozone is an air pollutant that damages human health, vegetation, and many common materials. It is a key ingredient of urban smog. In the stratosphere, we find the "good" ozone that protects life on earth from the harmful effects of the sun's ultraviolet rays.
Electromagnectic Spectrum
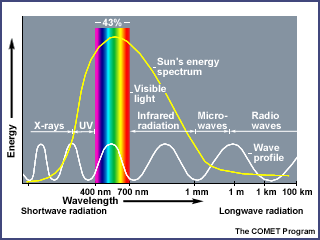
To understand how ozone is generated and the functions it serves in the earth's atmosphere, it is important to know something about the electromagnetic spectrum — the energy emitted from the sun. Electromagnetic energy is sometimes described as traveling in waves and sometimes as traveling in packets of energy referred to as photons.
Progressing from short wavelengths to long wavelengths, scientists have identified gamma rays, x-rays, ultraviolet radiation, visible light (between 400 and 700 nanometers), infrared radiation (heat), microwaves, and radio waves. Short wavelengths have more energy per photon than long wavelengths.
Ozone is constantly being formed in the earth's atmosphere by the action of the sun's ultraviolet radiation on oxygen molecules. Ultraviolet light splits the molecules apart by breaking the bonds between the atoms. A highly reactive free oxygen atom then collides with another oxygen molecule to form an ozone molecule. Because ozone is unstable, ultraviolet light quickly breaks it up, and the process begins again.
Ozone in the Stratosphere
Ozone and oxygen molecules in the stratosphere absorb ultraviolet light from the sun, providing a shield that prevents this radiation from passing to the earth's surface. While both oxygen and ozone together absorb 95 to 99.9% of the sun's ultraviolet radiation, only ozone effectively absorbs the most energetic ultraviolet light, known as UV-C and UV-B, which causes biological damage. The protective role of the ozone layer in the upper atmosphere is so vital that scientists believe life on land probably would not have evolved - and could not exist today - without it.
The term "shield" as a description of ozone in the stratosphere is a bit misleading because the molecules do not form an impermeable sphere around the earth. Ozone continuously breaks apart into its oxygen atoms and reforms as ozone molecules, so a particular ozone molecule doesn't last very long. The "shield" changes constantly, but the atmospheric chemical processes maintain a dynamic equilibrium that keeps the overall amount of ozone constant - that is, it would if humans did not contribute to the chemical processes.
About 90% of the ozone in the earth's atmosphere lies in the region called the stratosphere between 16 and 48 kilometers (10 and 30 miles) above the earth's surface. Ozone forms a kind of layer in the stratosphere, where it is more concentrated than anywhere else, but even there it is relatively scarce. Its concentrations in the ozone layer are typically only 1 to 10 parts of ozone per 1 million parts of air, compared with about 210,000 parts of oxygen per 1 million parts of air.
Ozone in the Troposphere
The other 10% of the ozone in the earth's atmosphere is found in the troposphere, which is the portion of the atmosphere from the earth's surface to about 12 km or 7 miles up. In the troposphere, ozone is not wanted. Ozone is even more scarce in the troposphere than the stratosphere with concentrations of about 0.02 to 0.3 parts per million. But even in such small doses, this molecule can do a lot of damage.
And just to confuse things even further, ozone in the troposphere is one of the greenhouse gases. As discussed in the Greenhouse Effect section, the naturally occurring greenhouse gases (including ozone) are what make earth habitable for life as we know it. But scientists are very concerned about the warming effects of increased greenhouse gases caused by human activity. So, in the troposphere, accelerated ozone levels deal us a double whammy - as a key ingredient in smog and as a powerful greenhouse gas.
Concluding Thoughts
Ozone is found in two different layers of the atmosphere - the troposphere and the stratosphere. The stratospheric ozone, or "good ozone," protects life on earth from harmful effects of the sun's UV rays. We have good reason to be concerned about the thinning of the ozone layer in the stratosphere. Tropospheric ozone, or "bad ozone," is an air pollutant that damages human health, vegetation, and many common materials. We have good reason to be concerned about the buildup of ozone in the troposphere. In this and the following sections, we will explore the various aspects of ozone to help your students better understand this complex, and sometimes seemingly contradictory, molecule. Although simplistic, the saying "Good up high and bad near by," sums up ozone in the atmosphere.











