Adapted from Mr. Dave Mastie at Pioneer High School in Ann Arbor, Michigan, who adapted this activity from Earth Science by Nancy E. Spaulding and Samuel N. Numowitz.
Type of Lesson: Hands-on activity
Time Needed: 15 minutes
Earth and Space Science, Grades 9-12: The Earth is a system containing essentially a fixed amount of each stable chemical atom or element. Each element can exist in several different chemical reservoirs. Each element on earth moves among reservoirs of solid earth, oceans, atmosphere and organisms as part of geochemical cycles.
Physical Science, Grades 9-12: Matter is made of minute particles called atoms, and atoms are composed of even smaller components…
Physical Science, Grades 9-12: Bonds between atoms are created when electrons are paired up by being transferred or shared. A substance composed of a single kind of atom is an element. The atoms may be bonded together into molecules or crystalline solids. A compound is formed when two or more kinds of atoms bind together chemically.
Unifying Concepts and Processes, Grades K-12: Models are tentative schemes or structures that correspond to real objects, events, or classes of events and that have explanatory power.
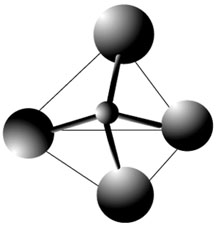
1. Each group is going to build a toothpick and raisin pyramid. First, use three toothpicks to make the triangle base of the pyramid. Toothpicks should be connected with 3 of the raisins (raisins are the vertices of the triangle).
2. Next, stick one toothpick in each of the vertex raisins in the base triangle so the toothpicks stand upright. Push the three toothpicks which are sticking up to a common middle point and connect them with the remaining raisin. Each group should now have a toothpick and raisin pyramid.
3. Straighten out the paperclip so that it looks like an "s" when layed flat. Hook the paperclip around the top of the pyramid.
4. If your cup wasn't filled by your teacher, place the dish soap or bubbles in the cup. Using the paperclip as a handle, dip the pyramid in the soap/bubbles. If the soap doesn't cover your pyramid, place a bit more soap in the cup.
5. Take turns using the straw to gently blow "an atom" in the middle of your tetrahedron structure. (By gently placing the straw in the middle of the soap that is clinging to the pyramid structure, and blowing gently with your straw, you should be able to get a small bubble that represents an atom).
6. Make sure you show your teacher your tetrahedron structure...then if there's time, see how big of a middle bubble you can make! Can you use your tetrahedron to blow normal bubbles? Have fun!
More than 90% of the minerals in the Earth's crust are members of the silicate family. In all silicates, the silica tetrahedron is the basic building block. And so it is important for students to understand the structure of the silica tetrahedron. The silica tetrahedron consists of 4 Oxygen atoms (raisins) bonded to 1 Silicon atom (bubble blown in middle of pyramid). This exercise allows students the chance to build a model of an extremely abstract concept (even drawing a tetrahedron can be difficult!).
It's suggested that students work in pairs for this exercise.
The raisins you use for this exercise can be used over and over again. Just warn your students not to eat any! You can use Dawn dish soap...the liquid that is in kids' bubbles works just as well. You can dilute the soap with water quite a bit to make the soap last longer (try 2 parts soap for 1 part water first, then dilute more if your tetrahedron holds together). Big plastic party cups work just fine for this exercise, but any cup with a wide enough mouth will work just fine as will big yogurt or sour cream containers.