Click on image for full size
Courtesy UCAR
Acid Rain
Acid rain is a general term used to describe different kinds of acidic air pollution. Although some acidic air pollutants return directly back to Earth, a lot of it returns in rain, snow, sleet, hail, mist or fog, hence the term acid rain. Most acidic compounds eventually get deposited because they are water soluble and therefore make their way into the water droplets in the atmosphere.
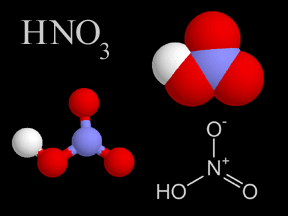
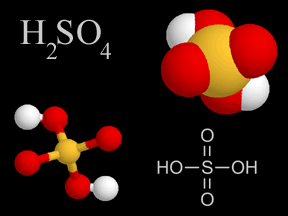
When power plants, factories, houses and cars release pollution into the atmosphere, it contains chemicals known as sulfur dioxide and nitrogen oxides. Sometimes, these chemicals return to the ground. This is known as dry deposition. The rest of the time, they mix with water (moisture) in the air to form acids. Once these acids have formed, they can be transported long distances by the wind before being deposited in rain, snow or hail. This is what we commonly call acid rain.
During the 1970s, scientists in Sweden and Norway began noticing that acid rain was damaging their trees and fresh water. Much of the acid rain was caused by pollution that was transported through the air from other countries, primarily the United Kingdom. After that, acid rain was understood to be an international problem.
Acid rain can have harmful impacts on the ecosystems in the environment. It acidifies the soil and water where it falls, damaging or even killing plants and animals. Surface water acidification can lead to a decline in, and loss of, fish populations and other aquatic species including frogs, snails and crayfish. Acid rain affects trees, usually by weakening them through damage to their leaves. Certain types of building stone, such as limestone and marble, can be gradually dissolved in acid rain.