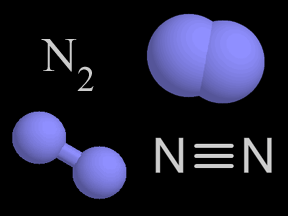Shown here are four representations chemists use for molecular nitrogen. In colored molecular models, nitrogen is traditionally shown in blue.
Click on image for full size
Windows to the Universe original artwork by Randy Russell.
Nitrogen
Nitrogen is a chemical element with an atomic number of 7 (it has seven protons in its nucleus). Molecular nitrogen (N2) is a very common chemical compound in which two nitrogen atoms are tightly bound together. Molecular nitrogen is a colorless, odorless, tasteless, and inert gas at normal temperatures and pressures.
About 78% of Earth's atmosphere is nitrogen. The strong triple-bond between the atoms in molecular nitrogen makes this compound difficult to break apart, and thus nearly inert. However, when nitrogen bonds do break, the resulting products are often highly reactive.
Nitrogen atoms are part of several types of pollutants. High temperature combustion in the presence of nitrogen gas, such as in automobile engines, can generate nitric oxide (NO) and nitrogen dioxide (NO2). Both gases are poisonous on their own, while they also play a role in the production of peroxyacetyl nitrate (PAN), a major component of smog, and nitric acid, which is part of acid rain.
Nitrogen gas can be used to manufacture ammonia (NH3), which is used extensively to produce chemical fertilizers.
Nitrogen is one of the most important elements in the chemistry of living creatures. For example, nitrogen is part of amino acids, the building blocks of proteins. The Nitrogen Cycle traces the path of nitrogen, in many different chemical forms, through the environment and living organisms. Certain microbes can take gaseous nitrogen from the air and convert it to ammonia, making it available to plants and other organisms in a process called "nitrogen fixation".
Last modified May 4, 2007 by Lisa Gardiner.
You might also be interested in:
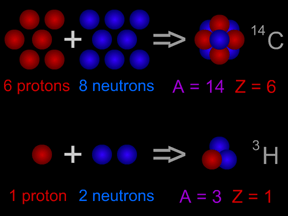
The atomic number of an atom tells us how many protons are in the nucleus of that atom. Why is that important? The chemical properties of an element are determined by the number of electrons in its atoms,
...more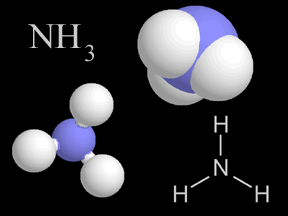
Most things around us are made of groups of atoms bonded together into packages called molecules. The atoms in a molecule are held together because they share or exchange electrons. Molecules are made
...more
What do smog, acid rain, carbon monoxide, fossil fuel exhausts, and tropospheric ozone have in common? They are all examples of air pollution. Air pollution is not new. As far back as the 13 th century,
...more
Air pollution comes from many different sources. Natural processes that affect air quality include volcanic activity, which produce sulfur, chlorine, and ash particulates, and wildfires, which produce
...more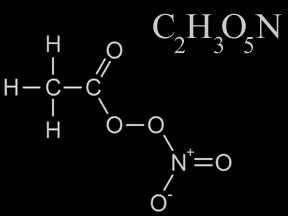
PAN (Peroxyacytyl nitrate) is a toxic chemical that is an important component of smog. PAN is a gas at normal temperatures and pressures. Its chemical formula is C2H3O5N. PAN molecules are composed of
...more
Smog is a type of air pollution. Smog is a mixture of smoke and fog, hence the name (SMoke + fOG = SMOG). Victorian-era London was famous for its thick smogs, which resulted from the city's frequent, naturally
...more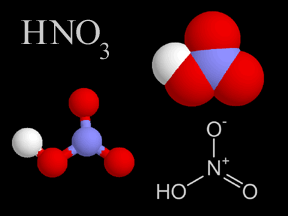
Nitric acid is a colorless, corrosive liquid and a toxic acid which can cause severe burns. Nitric acid consists of nitrogen, oxygen, and hydrogen atoms. Nitric acid, in its gas phase, is present in very
...more