The "Ivy Mike" test of the first Hydrogen bomb (1952)
Click on image for full size
Image courtesy of Carey Sublette, The EnviroLink Network
The Hydrogen Bomb
In the Hydrogen bomb the explosion of a nuclear fission charge (atomic bomb) produces the
temperature and density necessary for the fusion of Deuterium and Tritium (isotopes
of Hydrogen), resulting in a sudden release of a large amount of energy that produces
an even bigger explosion.
You might also be interested in:
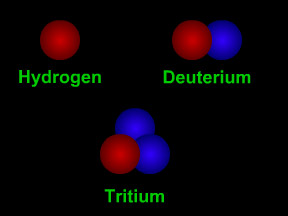
Isotopes are different "versions" of a chemical element. All atoms of an element have the same number of protons. For example, all hydrogen atoms have one proton, all carbon atoms have six protons, and
...more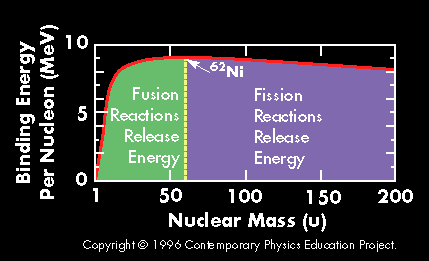
A plot of the binding energy per nucleon vs. atomic mass shows a peak atomic number 56 (Iron). Elements with atomic mass less then 56 release energy if formed as a result of a fusion reaction. Above this
...more
There are several experiments worldwide where the conditions for nuclear fusion reactions have been achieved in a controlled manner. The two main approaches that are being explored are magnetic confinement
...more
In the Hydrogen bomb the explosion of a nuclear fission charge (atomic bomb) produces the temperature and density necessary for the fusion of Deuterium and Tritium (isotopes of Hydrogen), resulting in
...more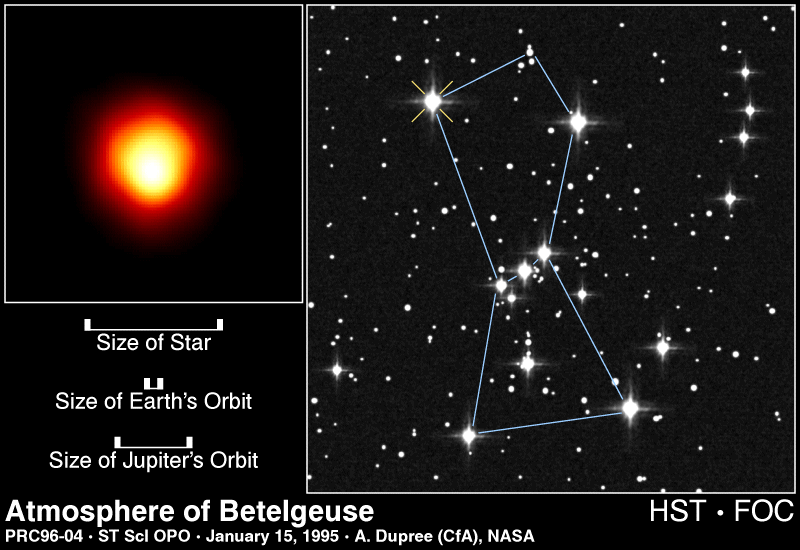
When the temperature in the core of a star reaches 100 million degrees Kelvin fusion of Helium into Carbon occurs (three Helium nuclei combine to form a nucleus of Carbon). In the same range of temperature
...more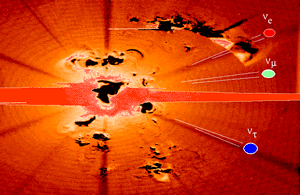
All of the matter and energy in the Universe was initially confined in a very small region until an enormous explosion occurred, causing the Universe to expand. This expansion continues today.
...more
The neutrino is an extremely light (and possibly massless) neutral particle. The neutrino belongs to the family of leptons, the particles that interact through the so-called weak force. For this reason
...more














