Click on image for full size
Courtesy of COMET program
Ozone in the Stratosphere
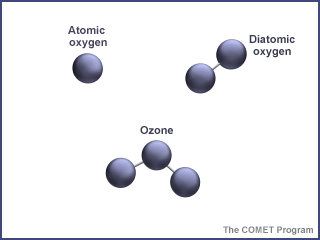
About 90% of the ozone in the Earth's atmosphere lies in the region called the stratosphere which is found between 16 and 48 kilometers (10 and 30 miles) above the Earth's surface. Ozone forms a kind of layer in the stratosphere, where it is more concentrated than anywhere else, but even there it is relatively scarce. Its concentrations in the ozone layer are typically only 1 to 10 parts of ozone per 1 million parts of air.
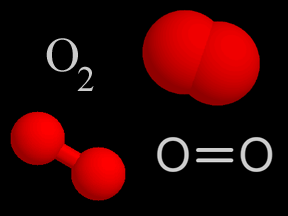
Ozone and oxygen molecules in the stratosphere absorb ultraviolet light from the Sun, providing a shield that prevents this radiation from passing to the Earth's surface. While both oxygen and ozone together absorb 95 to 99.9% of the Sun's ultraviolet radiation, only ozone effectively absorbs the most energetic ultraviolet light, known as UV-C and UV-B, which causes biological damage. The protective role of the ozone layer in the upper atmosphere is so vital that scientists believe life on land probably would not have evolved - and could not exist today - without it.
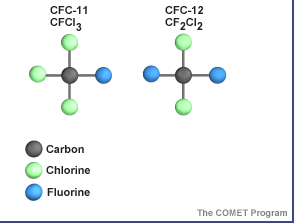
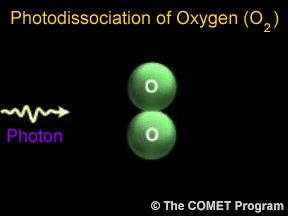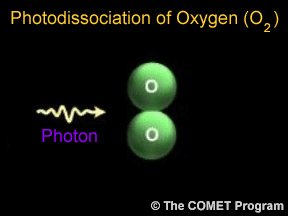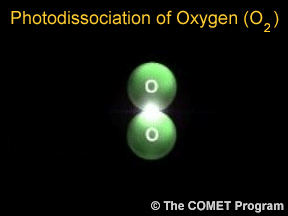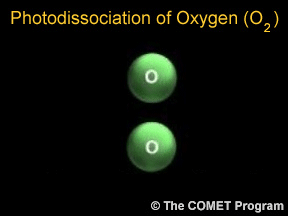
The term "shield" as a description of ozone in the stratosphere is a bit misleading because the molecules do not form an impermeable sphere around the Earth. Ozone continuously breaks apart into its oxygen atoms and reforms as ozone molecules, so a particular ozone molecule doesn't last very long. The "shield" changes constantly, but the atmospheric chemical processes maintain a dynamic equilibrium that keeps the overall amount of ozone constant - that is, it would if humans did not contribute to the chemical processes. There is compelling scientific evidence that ozone is destroyed in the stratosphere and that some human-released chemicals such as CFC’s are speeding up the breakdown of ozone in the atmosphere.
While the stratospheric ozone issue is a serious one, in many ways it can be thought of as an environmental success story. Scientists detected the developing problem, and collected the evidence that convinced governments around the world to take regulatory action. Although the global elimination of ozone-depleting chemicals from the atmosphere will take decades yet, we have made a strong and positive beginning. For the first time in our species' history, we have tackled a global environmental issue on a global scale.