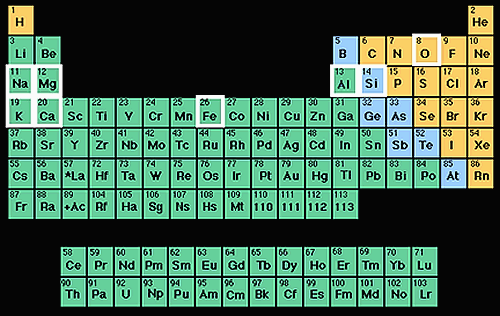
Periodic table of the elements with the common elements from the Earth's crust highlighted with white.
Click on image for full size
L.Gardiner/Windows to the Universe
Elements in the Earth’s Crust
Even though there are 92 elements that naturally occur, only eight of them are abundant in the rocks that make up the Earth’s outer layer, the crust. Together, these 8 elements account for 98.5% of the crust.
The 8 most abundant elements in Earth’s crust (by mass):
46.6% Oxygen (O)
27.7% Silicon (Si)
8.1% Aluminum (Al)
5.0% Iron (Fe)
3.6% Calcium (Ca)
2.8% Sodium (Na)
2.6% Potassium (K)
2.1% Magnesium (Mg)
The picture on the left shows where these elements are located within the periodic table. Together, the elements oxygen and silicon make up most of the Earth’s crust including silicate minerals such as quartz and feldspar.
Last modified November 13, 2007 by Lisa Gardiner.
You might also be interested in:
Everything you see around you is made of tiny particles called atoms, but not all atoms are the same. Different combinations of protons , neutrons and electrons make different types of atoms and these
...more
An element (also called a "chemical element") is a substance made up entirely of atoms having the same atomic number; that is, all of the atoms have the same number of protons. Hydrogen, helium, oxygen,
...more
Quartz is the second most common mineral in Earth’s crust. It is a member of the quartz group, which includes less common minerals such as opal, crystobalite, and coesite. Silica (Si) and Oxygen (O) are
...more
Feldspar is the most common mineral in the Earth’s crust, so you are very likely to find it in the rocks you collect! It is found it all of the three rock types, but is most common in intrusive igneous
...more
Canadian bedrock more than 4 billion years old may be the oldest known section of the Earth's early crust. Scientists at the Carnegie Institution of Washington and McGill University in Montreal used geochemical
...more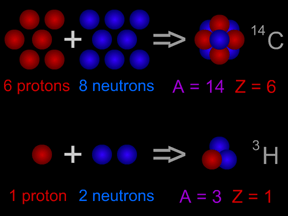
"Atomic mass" is a term physicists use to describe the size (mass) of an atom of a specific type. Since the nucleus of an atom contains nearly all (more than 99%) of an atom's mass, "atomic mass" is more-or-less
...more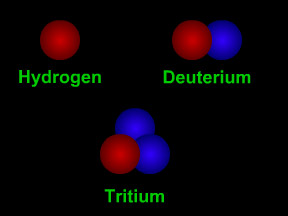
Isotopes are different "versions" of a chemical element. All atoms of an element have the same number of protons. For example, all hydrogen atoms have one proton, all carbon atoms have six protons, and
...more