This picture explains the idea of "atomic mass". The carbon atom (
14C) nucleus on the top has 6 protons plus 8 neutrons. It has an atomic mass of 14. Tritium (
3H), an isotope of hydrogen, is shown on the bottom. It has 1 proton plus 2 neutrons in its nucleus. Tritium has an atomic mass of 3.
Click on image for full size
Original artwork by Windows to the Universe staff (Randy Russell).
Atomic Mass
One way scientists measure the size of something is by its mass. Scientists can even measure very, very tiny things like atoms. One measure of the size of an atom is its "atomic mass". Almost all of the mass of an atom (more than 99%) is in its nucleus, so "atomic mass" is pretty much a measure of the size of the nucleus of an atom.
The nucleus of an atom is made up of protons and neutrons. Protons and neutrons are almost exactly the same size. If you add up the number of protons and neutrons in the nucleus of an atom, you get that atom's atomic mass. A simple hydrogen atom has just one proton and zero neutrons. Its atomic mass is 1. The most common kind of carbon atom has 6 neutrons and 6 protons. It has an atomic mass of 12.
All atoms of a certain element have the same number of protons. Oxygen atoms always have 8 protons; carbon atoms all have 6 protons. Most atoms come in different types called isotopes. Isotopes have different numbers of neutrons. The most common isotope of carbon has 6 neutrons and 6 protons. Its atomic mass is 12. A rare, radioactive isotope of carbon has 8 neutrons. Its atomic mass is 14 ( = 6 protons + 8 neutrons).
In chemistry, the number of protons in an atom is more important than the number of neutrons. Scientists call the number of protons the "atomic number". Normal atoms have the same number of electrons as protons. The number of electrons is the main thing that controls how atoms behave in chemical reactions. Scientists use the letter "Z" to stand for atomic number and the letter "A" to stand for atomic mass.
You might also be interested in:
An element (also called a "chemical element") is a substance made up entirely of atoms having the same atomic number; that is, all of the atoms have the same number of protons. Hydrogen, helium, oxygen,
...more
Isotopes are different "versions" of an element. All atoms of an element have the same number of protons. For example, all hydrogen atoms have one proton, all carbon atoms have 6 protons, and all uranium
...more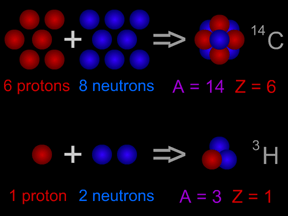
The nucleus of an atom has protons and neutrons in it. Each element (like carbon or oxygen or gold) has a different number of protons in its atoms. Scientists have a special name for the number of protons
...more
Carbon-14 is an isotope of the element carbon. All carbon atoms have 6 protons in their nucleus. Most carbon atoms also have 6 neutrons, giving them an atomic mass of 12 ( = 6 protons + 6 neutrons). Carbon-14
...more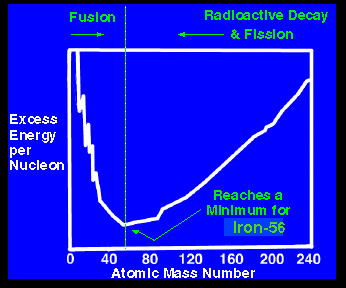
The diagram at the left gives the answer to that question. It shows the excess energy per nucleon plotted against the atomic mass number of an element. To illustrate what that means let's consider one
...more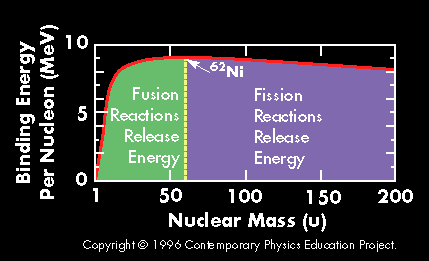
A plot of the binding energy per nucleon vs. atomic mass shows a peak atomic number 56 (Iron). Elements with atomic mass less then 56 release energy if formed as a result of a fusion reaction. Above this
...more
Looking for online content that can be used for a climate change education course or module? Pages linked below can be used to support an introductory climate change education for either a unit or a full
...more













