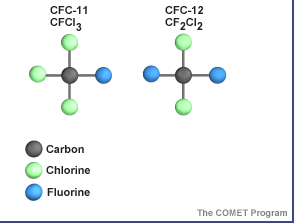
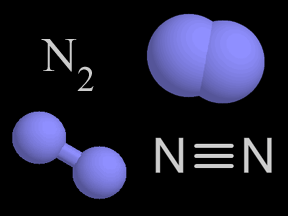Click on image for full size
Images courtesy COMET.
Photodissociation
Sometimes when a photon hits a molecule, the energy from the photon causes the molecule to break apart. Scientists use the term "photodissociation" for such events. Photodissociation plays a very important role in the chemistry of planetary atmospheres, including atmospheric chemistry here on Earth.
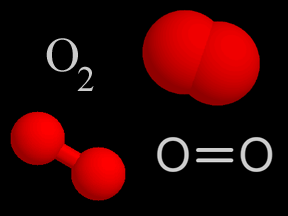
Photons of visible light can cause the photodissociation of some molecules. However, ultraviolet photons are even more likely to shatter molecular bonds because they carry more energy. The bonds between atoms within a molecule have characteristic strengths. For example, the triple bond of molecular nitrogen (N2) is stronger than the double bond of molecular oxygen (O2). This means that the bond between nitrogen atoms in N2 is harder to break, so a higher-energy photon is required to photodissociate N 2 than is the case for O2. Each such bond has a corresponding energy level that a photon must minimally carry to break that bond. If a photon carries more energy than the minimum required to break a given bond, it will be able to break the bond. If, however, it has less energy, it will not photodissociate the molecule.
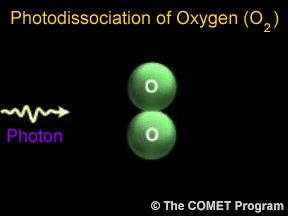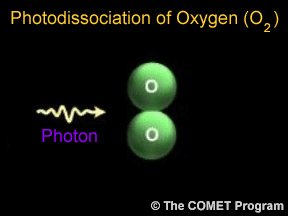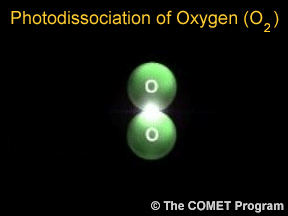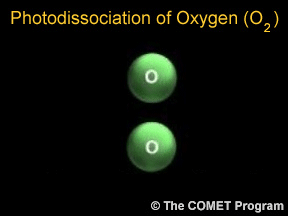
Photodissociation plays a key role in the formation of stratospheric ozone. Normal oxygen (O2) is split by photodissociation into two oxygen atoms. These oxygen atoms then combine with other oxygen molecules to form ozone (O3).
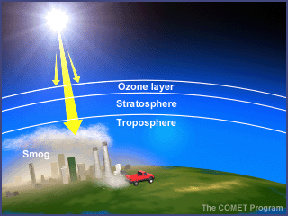
In the troposphere, photodissociation of nitrogen dioxide (NO2) produces nitric oxide (NO) and ozone. This reaction is one of the main sources of ozone in the troposphere. Photodissociation thus plays a key role in the formation of both good ozone (stratospheric ozone) and bad ozone (tropospheric ozone).
Photodissociation also plays a part in the formation of photochemical smog. A chemical called PAN (Peroxyacytyl nitrate) is a major constituent of smog. PAN forms when photodissociation alters Volatile Organic Compounds (VOCs). After a series of reactions, the transformed VOCs combine with nitrogen oxides to form PAN. PAN is a powerful respiratory and eye irritant that contributes to the unpleasant nature of smog.
Photodissociation is very common in the upper reaches of Earth's atmosphere. High energy photons from the Sun are prevalent there, and have a powerful influence of the chemistry of the upper atmosphere. This is fortunate for us, because much of the most damaging radiation from the Sun, especially in the ultraviolet part of the spectrum, is absorbed long before it reaches the ground where it can harm humans and other living things.