This image is a model of an atom
Windows to the Universe original animation by Randy Russell.
Related links:
Elements
Atomic Nucleus
What's a molecule?
A Matter of Scale - interactive showing the sizes of things, from very tiny to huge - from NSF
Atoms
Atoms are the smallest size pieces that elements come in. Each atom has a small but heavy nucleus at its center. A cloud of tiny, fast-moving electrons orbit around the nucleus. The nucleus contains at least one proton. The nucleus usually also has neutrons. Most of the time there are about as many neutrons as protons. A "normal" atom that doesn't have an electrical charge has the same number of electrons (which have negative electrical charges) and protons (which have a positive charge).
The animation of an atom on this page is NOT to scale. The distance between the nucleus and the electrons in an actual atom are much larger in comparison with the sizes of the protons, neutrons, and electrons. Most of an atom is empty space. Atoms are incredibly small. They range in size from about 60 to 500 picometers (a picometer is 10-12 meters, or one trillionth of a meter). The nucleus of an atom is about 100,000 times smaller than the whole atom. Almost all (more than 99%) of the mass of an atom is in the nucleus.
Sometimes two or more atoms "stick together" to form a molecule. Some molecules have atoms of just one type. For example, an oxygen molecule (O2) has two oxygen atoms. Other molecules combine different types of atoms. Methane (CH4) has one carbon atom and four hydrogen atoms.
You might also be interested in:
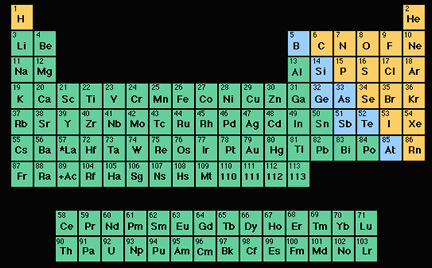
An element (also called a "chemical element") is a substance made up entirely of atoms having the same atomic number; that is, all of the atoms have the same number of protons. Hydrogen, helium, oxygen,
...more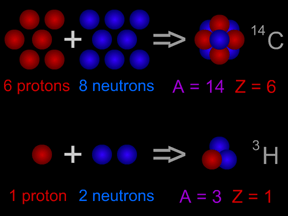
One way scientists measure the size of something is by its mass. Scientists can even measure very, very tiny things like atoms. One measure of the size of an atom is its "atomic mass". Almost all of the
...more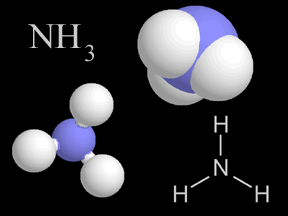
Most things around us are made of groups of atoms bonded together into packages called molecules. The atoms in a molecule are held together because they share or exchange electrons. Molecules are made
...more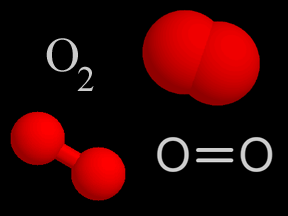
Oxygen (O2) is a kind of gas. A lot of the air you breathe is oxygen. That's a good thing, since we need oxygen to stay alive! About 4/5ths of the air in Earth's atmosphere is nitrogen (N2). Almost all
...more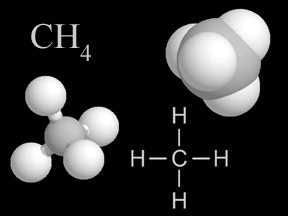
Methane is a kind of gas. There is a small amount of methane in the air you breathe. A methane molecule has carbon and hydrogen atoms in it. Methane is a greenhouse gas. That means it helps make Earth
...more
The nucleus of an atom has protons and neutrons in it. Each element (like carbon or oxygen or gold) has a different number of protons in its atoms. Scientists have a special name for the number of protons
...more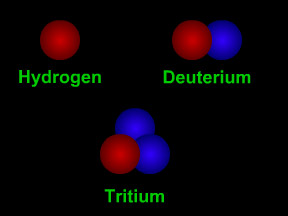
Isotopes are different "versions" of an element. All atoms of an element have the same number of protons. For example, all hydrogen atoms have one proton, all carbon atoms have 6 protons, and all uranium
...more













