Proton
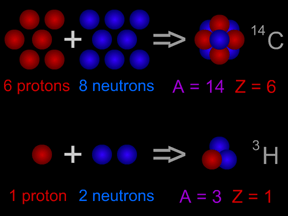
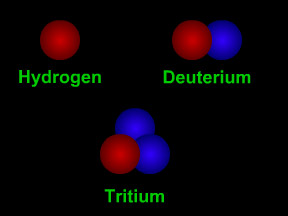
A proton is one of the most important types of subatomic particles. Protons combine with electrons and (usually) neutrons to make atoms. Protons are nearly the same size as neutrons and are much larger than electrons. A proton has a mass about 1,836 times greater than the mass of an electron, but the masses of protons and neutrons differ from each other by less than one percent. A proton has a mass of 1.6726 x 10-24 grams. Protons have a positive electrical charge, which is sometimes called the elementary charge or fundamental charge or a charge of +1. Electrons have a charge of the same strength but opposite polarity, -1. The fundamental charge has a strength of 1.602 x 10-19 coulomb. The nucleus of an atom is a combination of roughly equal numbers of protons and neutrons held together by the strong nuclear force. Clouds of electrons orbit the nucleus, attracted by the positive charges of the protons. Protons are baryons, a class of subatomic particles that also includes neutrons. Protons are composed of two up quarks and one down quark. A single electron orbiting a single proton is a simple hydrogen atom, the most abundant element in the Universe. Such hydrogen atoms often have their electron stripped away in a process called ionization, which leaves a lone proton. Such lone protons, also called hydrogen ions (H+), are very common. Because of their charges, these protons can be accelerated by electrical or magnetic fields to high energies, and can thus become a dangerous form of particle radiation. |
 Atomic Physics and Particle Physics
Atomic Physics and Particle Physics
 Fundamental Physics relevant to Space Weather
Fundamental Physics relevant to Space Weather
 A Matter of Scale - interactive showing the sizes of things, from very tiny to huge - from NSF
A Matter of Scale - interactive showing the sizes of things, from very tiny to huge - from NSF