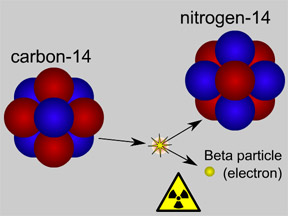
This picture shows radioactive decay of a carbon-14 atom. The carbon atom gives off a beta particle of radiation. The carbon atom turns into a nitrogen atom.
Click on image for full size
Original artwork by Windows to the Universe staff (Randy Russell).
Radioactive Decay
Some materials are radioactive. They give off radiation. When an atom of a radioactive substance gives off radiation, it becomes a new type of atom. This change is called radioactive decay.
There are two main types of radiation that can be given off during radioactive decay. The first is particle radiation. It includes alpha and beta particles as well as proton and neutron radiation. The second is electromagnetic radiation. It includes high energy gamma rays and X-rays.
Most elements come in different "versions", called isotopes. Some isotopes are radioactive. Carbon-14 is a radioactive isotope of carbon. Carbon-14 decays by emitting a beta particle. It becomes nitrogen-14, a different element! Some isotopes do not decay. They are called "stable" isotopes.
Different radioactive materials take different amounts of time to decay. Scientists use the idea of a half-life to describe this. The half-life of a radioactive material can be very short (less than a second) or very long (thousands of years) or anywhere in between. After one half-life, half of a sample of radioactive material has decayed. After another half-life, half of what was left decays.
You might also be interested in:
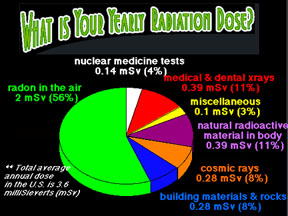
The text for this level hasn't been written yet. Please check the "Intermediate" or "Advanced" level of this page (click on the bar near the top of this page).
...more
Text for this level has not been written yet. Please see the "Intermediate" text for this page if you want to learn about this topic. To get to the "Intermediate" text, click on the blue "Intermediate"
...more
Text for this level has not been written yet. Please see the "Intermediate" text for this page if you want to learn about this topic. To get to the "Intermediate" text, click on the blue "Intermediate"
...more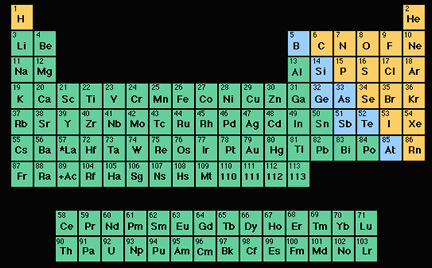
An element (also called a "chemical element") is a substance made up entirely of atoms having the same atomic number; that is, all of the atoms have the same number of protons. Hydrogen, helium, oxygen,
...more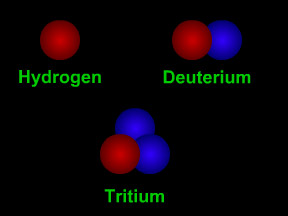
Isotopes are different "versions" of an element. All atoms of an element have the same number of protons. All hydrogen atoms have one proton, all carbon atoms have 6 protons, and all uranium atoms have
...more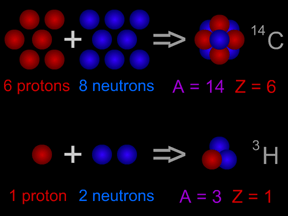
Carbon-14 is an isotope of the element carbon. All carbon atoms have 6 protons in their nucleus. Most carbon atoms also have 6 neutrons, giving them an atomic mass of 12 ( = 6 protons + 6 neutrons). Carbon-14
...more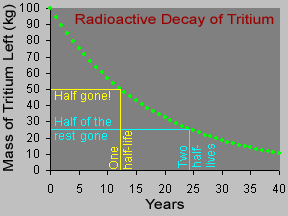
Some materials are radioactive. Their atoms give off radiation. When an atom gives off radiation, it turns into a different kind of atom. That is called radioactive decay. Some atoms decay very quickly,
...more